OBGYN

OBGYN
Contraindications to starting an IUD
Levonorgestrel and copper
Pregnancy or elevated beta-hCG (e.g. gestational trophoblastic disease)
Distorted uterine cavity and/or unexplained uterine bleeding
Pelvic infection and/or active sepsis
Active cervical and/or endometrial cancer
Levonorgestrel: Also applies to medroxyprogesterone implant and injections (see below)
Breast cancer within the past 5 years
Ischemic heart disease
Liver tumors and/or severe cirrhosis
Systemic lupus erythematosus with positive or unknown antiphospholipid antibodies
Copper: History of severe anemia or bleeding disorders (e.g. thrombocytopenia)
Contraindications to starting medroxyprogesterone
Implant (Nexplanon) and injection (Depo-Provera):
History of cerebrovascular disease
Morbid obesity (Nexplanon contraindicated if > 90 kg and Depo-Provera can result in weight gain)
All contraindications levonorgestrel IUD apply (see above)
Injection (Depo-Provera) only
Hypertension: Systolic > 160 mmHg and/or diastolic > 110 mmHg
Diabetes with vascular complications (retinopathy/nephropathy/neuropathy)
Severe thrombocytopenia
Note: The copper IUD is the only approved form of birth control for women with a history of breast cancer within the past 5 years and/or antiphospholipid antibody positive systemic lupus erythematosus.
Contraindications
Common
Hypertension: Systolic > 160 and/or diastolic > 110
Current medications: Rifampin, anticonvulsants, antiretrovirals
Actively breastfeeding and less than 42 days postpartum
Thrombosis risk:
Age > 35 years and an active smoker
History of superficial venous thrombosis
History of DVT/PE and not currently on anticoagulation
Systemic lupus erythematosus with positive or unknown antiphospholipid antibodies
Neurovascular: History of ischemic stroke, migraine with aura
Additional considerations
Breast cancer within the previous 5 years
Cardiovascular: Ischemic and/or valvular heart disease, diabetes with vascular complications (retinopathy/nephropathy/neuropathy)
Gastrointestinal/Hepatobiliary: History of bariatric surgery, active gallbladder disease, acute viral hepatitis, liver tumors and/or severe cirrhosis
Prescription Options
Ethinyl estradiol 0.03 mg and drospirenone 3 mg (Yasmin): Also used for acne, breast soreness, severe menstrual cramps, breakthrough bleeding
Ethinyl estradiol 0.035 mg and norgestimate 0.25 mg (Ortho-Cyclen)
May reduce depression, moodiness, irritability
Phasic version (Ortho Tri-Cyclen) increases progesterone dose every 7 days, i.e. 0.18 mg days 1-7, 0.215 mg days 8-14, 0.25 mg days 15-21
Ethinyl estradiol 0.03 mg and norethindrone acetate 1.5 mg (Loestrin): Also used for reduction of endometriosis symptoms
Counseling
Start first dose on the first Sunday following menstruation (if menstruation begins on Sunday, use an additional form of birth control x 1 week)
Missed doses
One missed dose (< 48 hours late): Take missed dose and resume dosing at normal time
Two or more missed doses (≥ 48 hours late)
Take most recently missed dose and discard previous doses
Use additional form of birth control x 1 week

See CDC Contraception Guidelines for contraindications before starting a combined hormonal contraceptive. Medications should not be started in smokers age > 35 or women with a history of migraine with aura.
Patient at < 20 WGA with h/o thyroid disease, diabetes mellitus, immunologic/thrombophilic disorders, alcohol/tobacco abuse, and previous aneuploid fetus presents with bleeding per vagina. Extreme BMI (see notes), partially dilated cervix with products of conception noted on exam.
Ultrasound shows uterine structural abnormality and embryo > 5 mm with no cardiac activity
Treatment (select one of the following):
Expectant management
Medical management: Administer misoprostol 800 mcg vaginally and repeat dose if complete expulsion does not occur by day 3
Surgical management: Schedule procedure (see below)
Schedule follow up in 2-4 weeks
Confirm negative urine beta-hCG
Evaluate for grief reaction vs. depression
Discuss modifying risk factors associated with spontaneous abortion
Notes
Etiology
50% of spontaneous abortions are due to chromosomal anomalies
Risk factors for spontaneous abortion:
Medical conditions: Hypothyroidism, hyperthyroidism, diabetes mellitus, autoimmune/thrombogenic conditions
Tobacco and/or excessive alcohol use
Extreme BMI, i.e. ≤ 18.5 or ≥ 40 kg/m^2
Structural abnormalities, e.g. uterine septum
Spontaneous abortion classification is based on os position and product of conception (POC) location
Missed: Closed os, POC within uterus, fetal demise
Dilated os
Inevitable: POC within uterus
Incomplete: POC within cervical canal
Complete: POC expelled from cervix
Treatment with intravaginal misoprostol has an 80% success rate
Patient presents with unintended, undesired pregnancy at < 11 WGA and desires medical abortion.
Following counseling about support services and adoption, patient elects to continue with medical abortion
Positive pregnancy test per urine beta-hCG: Obtain ABO/Rh status and dating ultrasound
Intrauterine pregnancy < 11 WGA confirmed by ultrasound
Rh negative and > 8 WGA: Administer Rhogam prior to procedure
Administer Mifepristone 200 mg now and misoprostol (Cytotec) 800 mcg buccally in 24 to 48 hours
Patient counseled about contraception options
Follow-up in two weeks for repeat urine beta-hCG and ultrasound to confirm elimination of intra-uterine pregnancy
Notes
Mifepristone is a progesterone antagonist
Misoprostol is a prostaglandin E1 analog
Antibiotic prophylaxis is no longer required for medical abortions
Patient with confirmed ectopic pregnancy at < 7 WGA presents for treatment. No history of active pulmonary disease, peptic ulcer disease, chronic liver disease, immunodeficiency, alcohol abuse. Patient not currently breastfeeding. Lungs clear to auscultation bilaterally and no hepatomegaly on exam.
Beta-hCG < 2,000 mIU/mL and Cr clearance > 50 mL/min
Gestational sac < 3.5 cm with no embryonic cardiac activity
Patient counseled about possibility for nausea/vomiting, abdominal/gastric pain, stomatitis following therapy
Administer methotrexate 50 mg/m^2 IM
Follow-up
Evaluate for 15% or greater beta-hCG decrease from day 4 to 7 s/p therapy
Continue weekly monitoring until beta-hCG reaches 0 mIU/mL
Notes
Absolute contraindications
Active pulmonary disease, chronic liver disease, hematologic dysfunction, peptic ulcer disease, alcohol abuse
Patient currently breastfeeding
Creatinine clearance < 50 mL/min
Efficacy
Success rate for starting beta-hCG < 1,000 mIU is 88% vs. 50% for starting beta-hCG > 3,000 mIU
15 to 20% of women will require 2 doses
Dose is calculated using body surface area
Ability to perform procedure varies by facility and local legal restrictions: Generally performed up to 19 WGA. However, our outpatient clinic performs to 11+6 WGA, our local Planned Parenthood performs to 15+6 WGA, and our local tertiary care center performs until 23+6 WGA. Check with local providers/facilities before counseling your patient.
Verify positive pregnancy test per urine beta-hCG prior to cervical preparation
Cervical preparation: Recommended in all pregnancies > 12 WGA as it reduces risk of cervical injury, uterine perforation, and incomplete abortion
Administer misoprostol 400 micrograms vaginally 3-4 hours prior to procedure
Patient informed that she may experience bleeding/cramping following misoprostol placement
If bleeding occurs during preparation, perform surgical abortion immediately
Administer analgesics, anxiolytics, and prophylactic antibiotics one hour prior to procedure, e.g.
Ibuprofen 800 mg PO
Diazepam 10 mg PO
Doxycycline 200 mg PO
Ask the woman to empty her bladder
Wash hands and use protective barriers
Perform a bimanual examination
Place the speculum
Perform cervical antiseptic preparation: Wipe cervix with non-alcoholic antiseptic solution starting at central cervical os and spiraling outward
Perform paracervical block using 1.0% lidocaine
Inject 1-2 mL where tenaculum will be placed (6 or 12 o’clock)
Stabilize cervix with tenaculum and inject 4 mL lidocaine at a depth of 2 cm at 4 locations along cervical/vaginal junction, i.e. at 2, 4, 8 and 10 o’clock
Assess cervical dilatation/dilate cervix if necessary
If greater than 12 WGA, perform amniotomy and aspirate amniotic fluid
Evacuate uterine contents (technique pending WGA)
For pregnancies < 12 WGA
The appropriate aspirator cannula size in millimeters is approximately the same as WGA (e.g. 12 mm cannula for 12 WGA)
The following signs during aspiration indicate that the uterus is empty: Red or pink foam in cannula with no more passage of tissue, gritty sensation as cannula passes over uterine surface, uterus contracts around cannula, patient feels intensified cramping or pain
For pregnancies > 12 WGA, procedure is termed dilation and evacuation (D&E)
Cannula size
> 12 WGA: Perform D&E with 14 mm cannula
> 16 WGA: Perform D&E with 16 mm cannula
Complete evacuation from lowest section of uterine cavity while holding cannula in horizontal position
Inspect the tissue
Products of conception (POC) should be visible including, villi, decidua, sac/membrane, and fetal parts after 9 WGA
Presence of grape-like villi in evacuated contents indicates likely molar pregnancy
If no POC are observed, consider incomplete abortion, spontaneous abortion, failed abortion, ectopic pregnancy, or anatomic abnormalities (e.g. bicornuate uterus)
Perform any concurrent procedures, e.g. cervical laceration repair or IUD placement
Recovery and discharge from the facility
Osmotic dilators are an alternative method for cervical preparation
Administration of NSAIDs (e.g. ibuprofen) does not interfere with action of prostaglandins (e.g. misoprostol)
Prophylactic antibiotics
Reduce risk of post-procedural endometritis
Alternative to doxycycline: Azithromycin 500 mg x 1 dose
Maximum lidocaine dose for a paracervical block: 4.5 mg/kg/dose
1 in 150 live births: Most common disorders include
Trisomy 21 (Down Syndrome): 1 in 800 live births
Trisomy 18 (Edward Syndrome): 1 in 7,000 live births
47 XXY (Klinefelter syndrome): 1 in 500 males
45 X (Turner syndrome): 1 in 20,000 females
Risk factors: Prior aneuploid fetus, increasing maternal age
Testing should be reviewed at first prenatal visit
First Trimester Combined Screen: 11+0 to 13+6 WGA
Screens for trisomy 21 only (85% detection rate)
Measurements/labs include
Nuchal translucency measurement (sonographer skill dependent)
Serum free beta-hCG
Total H-hCG
Pregnancy associated plasma protein A analyte (PAPP-A) levels
Quadruple Screen (AFP Tetra): 15+0 to 22+6 WGA
Screens for trisomy 21 (80% detection rate), trisomy 18, and open fetal defects
Labs include
Serum free beta-hCG
Inhibin A (placental protein)
Unconjugated estriol (uE3 - dominant estrogen produced during pregnancy)
Alpha fetoprotein (AFP - produced by developing liver and yolk sac)
Cell free DNA: 10+0 WGA to term
Information provided
All options tests for trisomy 21 (98% detection), trisomy 18, and trisomy 13, fetal sex
Additional information depends on the specific panel selected
Most commonly used in patients with advanced maternal age, i.e. > 35 years old at time of delivery
Verify insurance coverage before sending test
Stepwise model: Perform first trimester combined screen
Positive result → perform cfDNA or diagnostic testing
Negative result → perform Quad screen
Contingent model: Perform first trimester combined screen
High risk → perform cfDNA or chorionic villus sampling
Intermediate risk → perform Quad screen
Low risk→ no further screening
Educate family about condition
Discussion options, e.g. referral to genetics for further counseling, pregnancy termination, referral to a tertiary care center, perinatal hospice, adoption, etc.
More information: See ACOG Bulletin 163
Level 1: Required referral for general obstetrics consultation
Previous C-section: TOLAC counseling vs. schedule for repeat c-section
Level 3: Consult MFM for evaluation and potential co-management
Maternal indications
History of incompetent cervix, ≥ 3 miscarriages, and/or intrauterine fetal demise
Uncomplicated chronic or gestational hypertension
Hypertensive disease uncontrolled with medication and/or with abnormal lab values
Hyperthyroidism + urgent referral to endocrinology if uncontrolled
Gestational diabetes including GDMA1 and GDMA2
Seizure disorder on anticonvulsants
Positive antibody titers
Any titers ≥ 1:16
Positive Rh or Kell antibodies
Hemoglobinopathies including sickle cell
Potential congenital infections including HIV
Intrapartum: Preeclampsia with severe features
Fetal indications
Minor congenital abnormalities on ultrasound
Intrauterine growth restriction (growth scan +/- umbilical artery doppler)
Macrosomia (estimate fetal weight ≥ 4000 g)
Oligohydramnios and/or polyhydramnios
Level 4: Consult MFM and consider transfer of care
Maternal indications
Chronic/complicated cardiac, pulmonary, and/or renal disease
Pre-existing DM type 1 or uncontrolled DM type 2
Uncontrolled substance abuse
Placental abnormalities
Placenta previa after 30 WGA
Vasa previa
Fetal indications
Multiple gestation
Estimated fetal weight > 4500 g
Major congenital abnormalities
Intrapartum
Preterm labor or indication for c-section at ≤ 34 WGA
Preeclampsia with features of HELLP syndrome

Test Options
Non-stress test (NST)
Biophysical profile (BPP) includes
Non-stress test
Ultrasound measuring movement, tone, breathing, single deepest amniotic fluid pocket
Modified BPP: NST + single deepest pocket > 2 cm
Biophysical Profile: Two points for each of the following
NST with two accelerations within 20 minutes
One or more episodes of rhythmic fetal breathing movements of 30 seconds or more within 30 minutes (see video)
Three or more discrete body or limb movements within 30 minutes (see video)
One or more episodes of extension of a fetal extremity with return to flexion, or opening or closing of a hand
Amniotic fluid pocket exceeding 2 cm (see video)
Patient < 20 WGA with h/o motion sickness, migraine, and nausea presents with nausea and vomiting. Symptoms are worse in the morning but last all day. Denies abdominal pain, diarrhea. No fever, abdominal pain, abdominal tenderness on exam.
Initial treatment
Start ginger 250 mg q8h and pyridoxine (vitamin B6) 50 mg q8h
Trial of doxylamine (Unisom Orange) 25 mg before bed; may increase to 25 mg q8h
Nausea and vomiting refractory to initial treatment (advance through each of the following)
Promethazine (Phenergan) 25 mg q4h PRN and counsel patient about risk for extrapyramidal symptoms
Metoclopramide (Reglan) 10 mg q6h and counsel patient about risk for promotility effects, tardive dyskinesia
Ondansetron (Zofran) 4 mg q8h
Patient < 10 WGA with severe, refractory symptoms: Patient counseled that medication benefits likely outweigh risks
Patient > 10 WGA: Start ondansetron if patient fails promethazine and metoclopramide
Counseling
Patient advised to avoid large, high-protein meals
Patient advised that acupuncture therapy is not effective
Patient counseled against taking OTC scopolamine due to risk of fetal deformity
Patient counseled that pyridoxine must be taken 3 times daily every day to be effective
Patient counseled that nausea and vomiting typically resolves after 20 WGA
Notes
Differential diagnosis includes cholecystitis, gastroenteritis, GERD, and migraine headache
Continue ginger, pyridoxine, and doxylamine when starting promethazine, metoclopramide, or ondansetron
Ondansetron
Pregnancy category B
Crosses the placenta in the first trimester but has not been shown to cause adverse events in animal studies
Data for fetal safety in the first trimester are conflicting, but benefits likely outweigh risks in refractory cases
Pregnant patient with h/o fetal triploidy presents with severe nausea and vomiting. Greater than 5% weight loss noted during pregnancy. Symptoms refractory to combination of ginger, pyridoxine, doxylamine, and ondansetron. Tachycardia, orthostasis, dry mucous membranes on exam.
Obtain CBC, CMP, TSH, U/A, beta-hCG and evaluate for hypokalemia, elevated transaminases, hyperthyroidism, ketonuria, abnormally elevated beta-hCG
Obtain ultrasound to evaluate for multiple gestation and rule out molar pregnancy
Treatment
> 10 WGA: Start methylprednisolone 16 mg q8h x 3 days and then taper over 2 weeks
Consider trimethobenzamide 300 mg q6h
Hypovolemia
Start IV fluids with thiamine for dehydration
Consider admission for feeding tube placement
For more information, see ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia

36 y/o G1P0 at < 20 WGA with h/o HTN and pregestational DM presents for prenatal care. BP ≥ 140/90 on two occasions > 4 hours apart. Patient previously on an ACE inhibitor and atenolol; medications discontinued prior to pregnancy due to reduce IUGR risk. Family history includes preeclampsia. BMI > 30 kg/m^2. Dating ultrasound shows multiple gestation.
CBC, CMP, urine protein/creatinine all WNL
Monitor for IUGR: Refer for growth scan after 20 WGA if fundal height is 3 cm less than gestational age
Continue thiazide diuretic started before pregnancy
BP ≥ 150/100: Start one of the following medications and add a second agent if necessary
Nifedipine ER 30 mg qd (MDD 120 mg/day)
Labetalol 100 mg BID (MDD 200 mg BID)
Methyldopa 250 mg BID (MDD 250 mg BID when combined with other antihypertensives)
Notes
Definition: BP ≥ 140/90 on two occasions > 4 hours apart before 20 WGA
Risk factors include advanced maternal age (≥ 35 y/o at delivery), multiple gestation, chronic HTN, pregestational DM, family h/o preeclampsia, BMI > 30 kg/m^2
Chronic HTN is also diagnosed if elevated blood pressures persist past 12 weeks postpartum
Patient with no h/o HTN before 20 WGA presents for prenatal care. BP ≥ 140/90 on two occasions > 4 hours apart.
CBC, CMP, urine protein/creatinine all within normal limits
BP ≥ 150/100: Start one of the following medications and add a second agent if necessary
Nifedipine ER 30 mg qd (MDD 120 mg/day)
Labetalol 100 mg BID (MDD 200 mg BID)
Methyldopa 250 mg BID (MDD 250 mg BID when combined with other antihypertensives)
Perform in-office BP and non-stress test once weekly until delivery
Delivery
Induce if > 34 WGA with 1 or more of the following risk factors: Rupture of membranes, fetal size < 5th percentile on ultrasound, suspected abruptio placenta
Induce at 37 WGA in the absence of additional risk factors
45 y/o G1P0 twin gestation at > 20 WGA with h/o DM and renal disease presents with BP ≥ 140/90 on two occasions 4 hours apart. Denies headache, changes in vision. Reports preeclampsia during a previous pregnancy and h/o preeclampsia in a 1st degree relative. Elevated BMI, lungs clear to auscultation bilaterally, and no RUQ or epigastric pain on exam.
Labs
Spot urine protein/urine creatinine ratio > 0.3
Platelets > 100,000/mL, serum creatinine < 1.1 mg/dL, and liver transaminase levels less than 2 times the upper limit of normal
Consider antiphospholipid antibody assay if concern for autoimmune disease
Obtain weekly CBC, CMP
Imaging
Twice weekly in-office blood pressure and NST until delivery
Once weekly amniotic fluid index until delivery
Fetal growth ultrasonography every 3 weeks until delivery to monitor for IUGR
Start magnesium prophylaxis if severe features develop, i.e. headache that does not resolve with Tylenol, vision changes (blurring/flashing/scotoma), platelets < 100,000/mL, serum Cr > 1.1, AST or ALT > 2x upper limit of normal
Delivery
> 34 WGA with ≥ 1 risk factors (ROM, abnormal MFM results, size <5th percentile on U/S, suspected abruptio placenta): Start induction
No risk factors: Induce at 37 WGA
Postpartum
Observe for 72 hours
Follow-up appointment within 10 days of discharge
Patient instructed to call office if she develops H/A, changes in vision, N/V, CP, SOB, RUQ pain, edema
Aspirin 162 mg qd starting at 12 WGA during future pregnancies
Notes
Preeclampsia definition: Systolic BP ≥ 140 or diastolic BP ≥ 90 on two occasions 4 hours apart AND a spot urine protein/urine creatinine ratio > 0.3
Risk factors for preeclampsia include maternal age > 40 y/o, nulliparity, multiple gestation, preexisting diabetes mellitus, renal disease, history of preeclampsia, preeclampsia in a 1st degree relative, elevated BMI, and presence of phospholipid antibodies
45 y/o G1P0 twin gestation at > 20 WGA with h/o DM and renal disease presents with BP ≥ 160/110 on two occasions 15 minutes apart. Reports blurred vision with aberrations/scotoma, H/A not responding to analgesia. Crackles on lung exam concerning for pulmonary edema. Upper and lower extremity edema noted, 3+ patellar reflexes b/l.
Labs
Platelets < 100,000/microliter, serum creatinine > 1.1 mg/dL, and AST and ALT levels > 2 times the upper limit of normal
Obtain urine protein and urine creatinine
Consider obtaining serum LDH and uric acid levels
Admit to inpatient for monitoring
BP control
No bradycardia: Labetalol 20 mg IV <10min> 40 mg <10min> 80 mg <10min> hydralazine 10 mg IV <20min> emergency consult
Bradycardia present: Hydralazine 10mg IV <20 min> 10 mg <20min> labetalol 20 mg IV <10 min> labetalol 40 mg IV and an emergency consult
Seizure prophylaxis
No h/o myasthenia gravis: Magnesium 6g loading dose over 20 minutes
2g/hr maintenance while patellar reflex present
Check magnesium level upon loss of patellar reflex, RR < 12, or UOP < 30cc/hr
Administer 1g Ca gluconate if concern for magnesium toxicity
H/o myasthenia gravis: Levetiracetam 500 mg IV BID
Management
IVF < 100mL/hr, oral intake < 25 mL per hour
Place Foley catheter and monitor UOP; goal = 30mL/hr
Delivery at 24-34 WGA
Immediate delivery in cases of severe/resistant HTN, eclampsia, pulmonary edema, abruption
Two doses IM betamethasone 12 mg q24h prior to delivery in cases of PLT < 100,000, transaminase 2x ULN, IUGR, severe oligohydramnios, umbilical artery reversed end-diastolic flow, worsening renal function.
Deliver at 37 WGA if no contraindications
Continue mag x 24 h postpartum; monitor for 72h postpartum
Nifedipine if HTN continues postpartum (max dose 30 mg qAM + 60 mg qhs)
Postpartum
Continue magnesium sulfate at 2g/hr for 24h
Observe for 72h
F/u appointment within 10 days of discharge
Pt instructed to call office if she develops H/A, changes in vision, N/V, CP, SOB, RUQ pain, edema
Aspirin 81mg qd starting at 12 WGA during future pregnancies
Pt with h/o preeclampsia and no h/o trophoblastic disease presents with seizures at > 20 WGA. Seizures were preceded by H/A and visual changes. Convulsions lasted 60-90 sec and were followed by postictal state. No signs of injury on exam.
Pt placed on L side and intubation team notified
Administered 6g magnesium sulfate loading dose over 15 min
Continue magnesium at 2g/hr
Admit to L&D for continued observation
Pt with h/o preeclampsia with severe features presents with RUQ pain. Sudden onset of symptoms. Petechiae noted on exam.
CBC with platelet count < 50,000
Obtain CMP, fibrinogen, PT, PTT
Platelets < 20,000; administer platelets prior to attempted vaginal delivery and consider regional anesthesia if repeat platelets > 100,000
Continue magnesium until 24-48h postpartum
Pt with h/o repeat miscarriage, high-dose neck radiation, DM1 and hypothyroidism presents s/p positive pregnancy test. Reports recent fatigue, weight gain, decreased exercise capacity, and constipation. Bradycardia, dry skin, and hair loss noted on exam.
Repeat urine pregnancy test
Obtain TSH, free T4 q4 weeks until 20 WGA; measure again at 24-28 and 32-34 WGA
Pt instructed to increase levothyroxine by two doses/week prior to dose titration per TSH, free T4
Titrate levothyroxine to trimester-appropriate TSH
1st: 0.1-2.5
2nd: 0.2-3.0
3rd: 0.3-3.0
Pt counseled about importance of levothyroxine adherence to reduce risk of miscarriage/preterm birth
Pt counseled about increased risk for hypertensive disorders and abruption
Pt counseled about risk for postpartum thyroiditis and how to recognize symptoms of hyper/hypothyroidism
Resume pre-pregnancy levothyroxine dose postpartum
Pt with h/o goiter presents s/p positive pregnancy test. Reports increased nervousness, heat intolerance, and diarrhea. Tachycardia, HTN, sweating, tremor, and proximal muscle weakness on exam.
Labs show low TSH, elevated free T4
Propylthiouracil 50 to 200mg BID during 1st trimester
Methimazole 5-20mg BID during 2nd and 3rd trimester
Obtain TSH and free thyroxine labs q2 weeks until serum free thyroxine in upper 1/3 of normal range; test weekly after 32 WGA
Pt counseled about importance of medication adherence to reduce fetal anomalies, heart failure, placental abruption, preeclampsia, and preterm delivery
36 y/o G2P1001 with h/o previous GDM/macrosomia in pregnancy, physical inactivity, non-European heritage, and a first degree relative with diabetes mellitus type 2 presents for prenatal care. Weight gain > 11 lbs since age 18 years and BMI > 25 kg/m^2.
Initial visit
Positive urine beta-hCG test in office
BMI > 25 kg/m^2 + 1 risk factor (see notes below): Obtain HbA1c
GDM screening at 24-28 WGA with 50 g 1 hour glucose tolerance test
Patient instructed to fast for 8 hours prior to test
Goals (mg/dL): Fasting < 95, 1 hour < 140
Failed 1 hour test (any value greater than goal): Schedule 100 g 3 hour test
HbA1c > 6.4% or positive 3 hour glucose test: Patient advised to monitor fasting (goal < 95 mg/dL) and 1 hour postprandial (goal < 140 mg/dL) levels.
Nutrition and weight management
Advised to maintain total pregnancy weight gain < 40 lbs
Recommend 30 minutes moderate aerobic exercise daily
Refer for nutrition consult
Start metformin 500 mg daily if > 50% home values exceed goals and titrate to 1,000 mg BID per fingersticks. For additional control, continue metformin and
Start insulin glargine 0.3 u/kg daily and increase dose by 10% weekly until ≥ 5 daily fasting fingersticks are < 95 mg/dL or patient experiences hypoglycemia (fingerstick < 70 mg/dL)
Elevated postprandial fingersticks despite maximum glargine: Start insulin aspart 0.1 u/kg TID premeal
Antenatal Testing and Delivery
Consult Maternal Fetal Medicine at time of diagnosis
Obtain growth ultrasound at 37 WGA and offer schedule c-section for estimated fetal weight > 4,500 g
Induction
GDMA1: Offer at 39+0 WGA and perform at 41+0 WGA if still pregnant
GDMA2: Schedule induction of labor at 39 WGA due to increased risk for stillbirth
Postpartum
Obtain fasting glucose at 6 and 12 week follow-up appointments
Screen for DM using HbA1c every 3 years following delivery
GDMA1
Obtain fingersticks q4 hours
Fluids: Fingerstick (mg/dL)
≥ 70: Normal saline at 125 cc/hr
< 70: D5NS at 125 cc/hr
Well controlled GDMA2
Obtain fingersticks q2 hours in latent labor and q1 hour in active labor
Fluids: Fingerstick (mg/dL)
≥ 100: Normal saline at 125 cc/hr
< 100: D5NS at 125 cc/hr
Glucose control
Initial: Continue oral and basal insulin, hold mealtime insulin
Two fingersticks > 150 mg/dL: Convert to poorly controlled protocol (see below)
Poorly controlled GDMA2
Obtain fingersticks q1 hours
Start D5NS at 125 mL/hr
Start insulin drip
Initial fingerstick: < 80 mg/dL (0 u/hr), 80-120 (0.5), 121-140 (1), 141-180 (1.5), 181-220 (2), > 220 (2.5)
Adjust per protocol
Risk factors for GDM
Age > 35 years
Past medical history: GDM, macrosomia in pregnancy
Family history: Non-European heritage, first degree relative with hypertension and/or diabetes mellitus
Physical exam: Weight gain > 11 lbs since age 18 years, BMI > 25 kg/m^2
Three hour glucose tolerance test
Positive if two values values > goals
Goals (mg/dL): Fasting < 95, 1 hour < 180, 2 hour < 155, 3 hour < 140
GDMA1 vs. GDMA2
GDMA1: Glucose controlled with lifestyle alone
GDMA2: Medication required to control glucose
Management
There is no strong evidence showing that dietary counseling improves outcomes
Medications
Oral medications safe in pregnancy include metformin and glyburide
Pharmacologic management decreases risk for maternal preeclampsia, large for gestational age infants, operative delivery, and shoulder dystocia
26 y/o G1P0 at 28 WGA with h/o atopy presents with erythematous papules and nodules on extensor surfaces of the extremities.
Obtain CMP, total/direct bilirubin, bile acid level, and prothrombin time to rule out alternative etiologies
Hydrocortisone valerate 0.2% ointment (group 4 corticosteroid) and loratadine 10 mg daily for symptom control
Pt counseled that condition does not adversely affect pregnancy outcome
![Polymorphic Eruption of Pregnancy. Image by Heykerriann at English Wikipedia [Public domain].](https://images.squarespace-cdn.com/content/v1/5acb26595ffd203815e5314f/1572866355845-EAFVWS2RPEBO4OZV3RL9/PUPPP.jpg)
Polymorphic Eruption of Pregnancy. Image by Heykerriann at English Wikipedia [Public domain].
26 y/o G1P0 at 28+ WGA presents with intensely pruritic rash. Rash first appeared on abdomen along striae lines. Urticarial plaques and papules present on exam.
Obtain CMP, total/direct bilirubin, bile acid level, and prothrombin time to rule out alternative etiologies
Consider lesion biopsy if concerned for pemphigoid gestationis or pustular psoriasis
Hydrocortisone valerate 0.2% ointment (group 4 corticosteroid) and loratadine 10 mg daily for symptom control
Patient counseled that condition does not adversely affect pregnancy outcome
26 y/o G1P0 at 28 WGA with h/o gallstones presents with pruritus. Pruritus is worse at night and most severely affects the palms and soles. Jaundice, excoriations, and prurigo nodules on exam.
Labs
Obtain CMP, total/direct bilirubin, prothrombin time
Serum bile acid levels > 16 mcg/mL indicate increased risk for adverse fetal outcomes
Medications
Start loratadine 10 mg daily for pruritus
Consider ursodiol [Actigall] 300 mg BID for
Consults
Refer to Maternal Fetal Medicine for evaluation
34 WGA: Start twice weekly monitoring with NST on Mondays and and modified BPP (NST + single deepest pocket) on Thursdays
Schedule for induction of labor at 37 WGA
Patient counseled that
Condition increases risk for premature delivery and intrauterine fetal demise
Pruritus generally resolves after delivery
Liver function will be retested 6-8 weeks after delivery
Notes
Rare condition
Onset is generally occurs during the second or third trimester
The rash present is secondary to excoriation and not associated with increased bile acid levels
Pregnant pt at >24 WGA with h/o smoking and intrauterine growth restriction (IUGR) presents with perceived decreased fetal movement during. Reports laying on side and counting fewer than 10 kicks during the past two hours. Pt took sedating medications including a benzodiazepine and non-benzodiazepine hypnotic shortly before onset of decreased fetal movement. Denies vaginal bleeding/discharge, contractions. Reduced fundal height and no fetal movements palpated on exam.
Fewer than 10 kicks in two hours: Perform non-stress test and biophysical profile within 24 hours
Recurrent decreased fetal movement:
<37 weeks: Perform a non-stress test and ultrasound twice weekly
37 to 39 weeks: Consider induction
>39 weeks: Deliver infant
Pt counseled that
Fetal activity may vary throughout the day and is generally greatest in the late evening
Perceived movement may decrease in the third trimester as room for fetal movement decreases
Quickening (first perceived fetal movements) may occur between 13 and 25 WGA
Factors that may contribute to perceived decreased fetal movement
Decreased maternal perception of movement due to
Early or late gestational age
Maternal position, e.g. standing
Maternal distraction
Sedating medications including benzodiazepines and non-benzodiazepine hypnotics (e.g. zolpidem)
Patients with decreased fetal movement
Should contact a provider if they experience no fetal movement for 2 hours
Are at greater risk for stillbirth; however, intervention may not change outcomes and increases c-section rates (debate exists about the evidence)
Kick counts
No strong evidence that it improves outcomes
Should not be performed in the supine position
If performed, fewer than 10 kicks in 2 hours should prompt further evaluation
Biophysical profile
Singleton white pregnancy presents with estimated fetal weight and abdominal circumference <10th percentile on initial anatomic ultrasound. Mother reports h/o HTN, GDM, thrombophilia, smoking, cocaine use, and IUGR affecting a previous pregnancy. Current pregnancy complicated by vaginal bleeding during 1st trimester and recent febrile illness. Fundal height less than predicted by current weeks gestational age (WGA).
LMP, initial dating ultrasound, and calculated due date reviewed and found to be accurate
Labs
Rule out fetal aneuploidy and obtain cell free DNA (cfDNA) if initial testing is non-reassuring
Suspicion of rubella, varicella, CMV, toxoplasmosis infection: Evaluate for maternal seropositivity
Consider evaluation for antiphospholipid syndrome
Imaging
Obtain biophysical profile (BPP)
Detailed fetal anatomic survey reveals abnormal fetal anatomy, umbilical cord structure, placental structure
Serial anatomic surveys show
Fetus failing to progress along normal growth curve
Reduced abdominal circumference growth velocity
Continued management
Monitor with once weekly NST and growth scan; consider reducing frequency to once every two weeks if results are reassuring
Abnormal BPP: Refer for umbilical artery Doppler velocimetry; consider administering antenatal corticosteroids and delivering immediately for
Abnormal ductus venosus
32+ WGA with reversed diastolic flow
34+ WGA with absent diastolic flow
Plan for induction no later than 39 WGA and send arterial and venous cord blood samples s/p delivery
Pt counseled that with the exception of stopping smoking and cocaine use, there is nothing she can do to alter fetal growth pattern
Normal vs. abnormal growth
Twin, triplet, etc. gestations and (often) non-white babies in the U.S. follow non-standard growth curves
IUGR is technically defined as <10th percentile, but fetuses in the 5th to 10th percentile with no other abnormalities are more likely to be constitutionally small vs. growth restricted
True growth restriction is more likely in cases with an abnormal head circumference:abdominal circumference ratio
Growth restricted fetuses
Potential etiologies include genetic abnormalities, placental insufficiency, infectious diseases, maternal health conditions, and exposure to teratogens and/or other noxious substances
At greater risk perinatal morbidity and mortality
Intervention
Cell free DNA allows for fetal karyotyping
Early delivery based on Doppler velocimetry results may reduce stillbirths while increasing neonatal deaths. Long term outcomes may also not be affected. Research is ongoing.
Patient with h/o positive pregnancy test presents with first trimester bleeding. No vaginal, cervical, or hemorrhoid bleeding noted on exam.
U/S shows embryonic cardiac activity, blood present between chorion and uterine wall
Patient informed that risk for spontaneous abortion is 9% given presence of cardiac activity
Schedule for f/u in 1 week
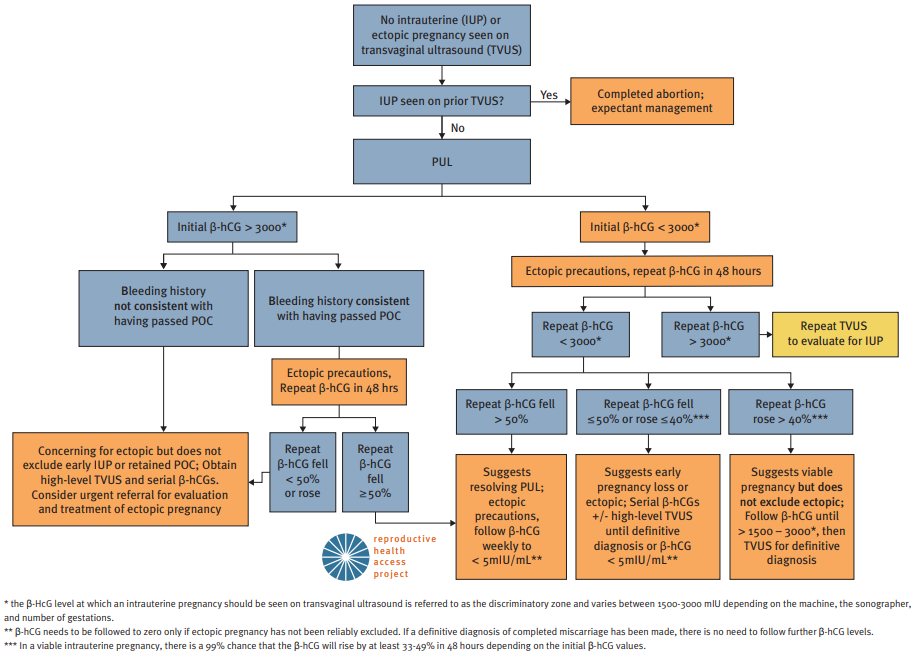
Initial evaluation of First Trimester Bleeding in Pregnancy of Unknown Location (PUL). Source: www.reproductiveaccess.org. Diagnosis and treatment algorithm is also available through the Reproductive Health Access Project.
Patient with h/o previous ectopic pregnancy, smoking, pelvic inflammatory disease (PID), and tubal surgery presents with abdominal pain and bleeding. LMP 6 weeks ago with IUD in place. No adnexal tenderness, rebound tenderness, cervical motion tenderness, or tissue lacerations. No products of conception present on speculum exam.
Obtain urine pregnancy test, CBC, blood type, and Rh status
Initial beta-hCG > 1500 mIU and increased < 50% after 48 hours
Trans-vaginal ultrasound (TVUS)
Failed to visualize intrauterine gestational sac and/or embryonic pole
Adnexal mass present
Treatment
Rh negative: Administer RhoGam
Medically stable: Discuss expectant management vs. methotrexate termination
Repeat beta-hCG in 4 to 7 days to ensure decrease of 15%
Failure of beta-hCG to decrease by 15%: Refer for surgical intervention
Ongoing pelvic pain, unstable vital signs, signs of intraperitoneal bleeding and/or failure of medical management: Refer for laparoscopic surgical intervention
Notes
Affects 1-2% of pregnancies
Major risk factors include previous tubal surgery (OR 21.0), previous ectopic pregnancy (OR 8.3), IUD (OR 5.0), h/o PID (OR 3.4), and smoking (OR 1.7-3.9)
Physical exam
Ectopic pregnancies often bleed even though they are not ruptured.
Rebound abdominal pain or cervical motion tenderness may indicate hemoperitoneum (surgical emergency)
Beta-hCG
Increases by 50% in 48 hours in 99% of viable pregnancies
For values >1500 mIU, an intrauterine pregnancy should be visible on U/S (note that the flow chart below uses >3000 mIU as a threshold)
For values <1500 mIU, repeat beta-hCG every 48 hours until a trend is established
For intrauterine pregnancies, TVUS should visualize a gestational sac with a yolk sac by 6 WGA
Consider laparoscopy if diagnosis is not clear within 10 days
Pt with presents with first trimester bleeding. No vaginal, cervical, or hemorrhoid bleeding noted on exam.
Obtain baseline beta-hCG, CBC, CMP, TSH
U/S showing snowstorm appearance of amorphous material
Schedule prompt surgical evaluation
Rh negative: administer 250 IU anti-D immunoglobulin s/p surgical evacuation
Pt to f/u s/p surgical evacuation for serial beta-hCG on days 1, 7, 14, and 21
Prescribe combined hormonal OCP during f/u provided no contraindications exist
Pt with h/o placenta previa before 20 WGA presents with late-pregnancy painless vaginal bleeding. Denies recent placement of object(s) in vagina. VSS. Bright red blood per os observed on speculum exam; no cervical abnormalities noted.
Obtain CBC, fibrinogen, PT, PTT, blood type, antibody screen; G/C if delivery is not imminent
Obtain fetal NST
U/S to evaluate for placenta within 2cm of internal cervical os at > 28 WGA
<37 WGA with preterm contractions; administer tocolytic
<34 WGA with preterm contractions; administer corticosteroids
Repeat U/S at 36 WGA to determine appropriate mode of delivery and r/o placenta accreta due to previous c-section
Perform amniocentesis at 36-37 WGA to document pulmonary maturity
Pelvic rest advised
Pt with h/o HTN, thrombophilia, tobacco/stimulant abuse presents with late-pregnancy vaginal bleeding and abdominal pain. Denies recent placement of object(s) in vagina. VSS. Bright red blood per os observed on speculum exam; no cervical abnormalities noted.
Obtain CBC, fibrinogen, PT, PTT, blood type, antibody screen; G/C if delivery is not imminent
Obtain fetal NST
U/S to evaluate for blood between placenta and myometrium
Rh neg.; Kleihauer-Betke test and administer Rhogam
<34 WGA with minor abruption; administer tocolytic, corticosteroids
Pt to be admitted for chronic monitoring if abruption recurs
Pt advised to stop tobacco/stimulant use
Pt with late-pregnancy painless vaginal bleeding that started s/p SROM. Denies recent placement of object(s) in vagina. VSS. Bright red blood per os observed on speculum exam; no cervical abnormalities noted.
Obtain CBC, fibrinogen, PT, PTT, blood type, antibody screen; G/C if delivery is not imminent
Obtain fetal NST; if reassuring, analyze vaginal vault blood for fetal cells/hemoglobin (Apt test)
U/S to evaluate for vasa previa
Screen for vasa previa at 37-38 WGA during future pregnancies
Pt with h/o coagulopathy presents with > 500mL EBL s/p vaginal delivery. Poor uterine tone, trauma, non-intact placenta noted on exam.
Obtain 16 gauge IV access; administer LR at 2:1 ratio of EBL
Initiate fundal message, Pitocin 40 IU/L IV
Cytotec (misoprostol) 1000mg rectally
No h/o asthma: Hemabate/Carboprost (15-methyl PGF2 alpha) 250mcg; repeat q15min, max 8 doses
No HTN: Methergine 0.2mg IM; repeat q2-4 hours
If bleeding continues despite medical therapy, obtain STAT labs with coags & fibrinogen; call blood bank and OB service
G1P0 at ≥ 39 WGA with h/o GDM and new onset preeclampsia presents for induction of labor (IOL). Gravid uterus; vertex per Leopold’s and ultrasound.
Obtain GBS swab results prior to induction
ACOG Induction of Labor Safety Checklist reviewed before induction
Bishop score < 6: Initiate cervical ripening prior to IOL
Mechanical cervical dilation (select one)
Laminaria japonica; risk of peripartum infection discussed with pt
Foley balloon (14-26 French)
No h/o c-section: PGE analogues
Misoprostol (Cytotec, PGE1) 25 mcg intravaginally q4h for 6 doses
Dinoprostone (Cervidil, PGE2) 10 mg insert; recheck after 12 hours
Other
H/o C-section: Start low dose pitocin at 0.5 mU/min and increase 1 mU every 30 minutes
Consider amniotomy in addition to Pitocin to reduce induction-to-delivery interval
Pt advised to try nipple stimulation
Bishop ≥ 7: Start Pitocin 2 mU/min; increase by 2 mU/min every 30 minutes to achieve contractions q3 minutes (maximum 40 mu/min)
Fetal head engaged and not ballotable: Consider amniotomy
Stop pitocin if any of the following are observed; restart at 2 mU/min and retitrate once resolved
Tachysystole, i.e. > 5 contractions/10 min averaged over 30 min
Repeat decelerations on fetal heart tracing
Stop induction due to failure to progress if no appreciable cervical change observed after 24 hours
Elective induction
Do not perform before 39 WGA; research into benefit between 39 and 41 weeks is ongoing
Cervical ripening vs. beginning pitocin at Bishop score 6-7 is provider and patent dependent
IOL indications
Abruptio placenta
Chorioamnionitis
Fetal demise
Gestational HTN
Preeclampsia
Post term pregnancy
Maternal medical condition (DM, renal disease, chronic pulmonary disease, chronic hypertension, antiphospholipid syndrome)
Fetal compromise (severe fetal growth restriction, isoimmunization, oligo/polyhydramnios)
IOL contraindications
Vasa previa or complete placenta previa
Transverse fetal lie
Umbilical cord prolapse
Previous classical c-section
Active genital herpes infection
Previous myomectomy entering endometrial cavity
26 y/o G2P0101 at 34 WGA with h/o preterm delivery presents s/p a sudden gush of fluid per vagina. Denies sexual intercourse during the previous 24 hours. Pregnancy complications include smoking, gonorrhea, and chlamydia infection. Pooling, cervical dilation/effacement, and fluid discharge through the cervix noted on sterile speculum exam.
Send GBS swab and gonorrhea/chlamydia cervical swab obtained during exam
Positive ferning and nitrazine paper test
Reassuring non-stress test (NST)
GBS status unknown
5mU penicillin G loading dose followed by 3mU q4h
Penicillin allergy: See alternative GBS prophylaxis options, UNC-CH GBS Algorithm
≥ 34 WGA: Start induction and plan for delivery
26 y/o G2P0101 at 34 WGA with h/o preterm delivery < 18 months prior presents with contractions every 5-10 minutes. Current pregnancy complications include smoking, multiple UTI, gonorrhea/chlamydia, GDMA2, cervical length < 2.5 cm. Completed course of hydroxyprogesterone caproate (Makena) 250mg IM weekly from 16-34 WGA with no missed doses. Cerclage was contraindicated due to multiple gestation. BMI < 20 kg/m^2 with 3 cm cervical dilation and suspected rupture of membranes on sterile speculum exam.
Labs
No intercourse within past 48 hours: Consider fetal fibronectin
Perform GBS testing
Obtain gonorrhea/chlamydia NAAT urine, urinalysis, and urine culture
Treatment
GBS status presently unknown
5mU penicillin G loading dose followed by 3mU q4h
Penicillin allergy: See alternative GBS prophylaxis options, UNC-CH GBS Algorithm
Administer two doses betamethasone 12 mg IM 24 hours apart
No h/o myasthenia gravis: Administer 6g magnesium loading dose then 2g/hr for tocolysis and CP risk reduction
Patient encouraged to hydrate PO
26 y/o G2P1001 < 37 WGA with h/o positive GBS status during previous pregnancy, GBS bacteriuria during current pregnancy presents in labor. Membranes ruptured > 18 hours ago. Records indicate positive GBS test within previous 5 weeks.
Start GBS ppx for any of the following:
GBS positive during previous pregnancy
GBS bacteriuria and/or positive GBS culture during current pregnancy
Culture not performed or > 5 weeks from negative culture with any of the following:
< 37 WGA
ROM ≥ 18 hrs
Maternal temperature > 38 C
Agents in order of preference:
Penicillin G 5 million units IV loading dose then 2.5 to 3 million units IV q4 hours until delivery
PCN allergy not no h/o anaphylaxis: Cefazolin 2g IV initial dose then 1g IV q8 hours until delivery
PCN allergy with h/o anaphylaxis:
Sensitive to clindamycin and erythromycin: Clindamycin 900 mg IV q8 hours until delivery
Vancomycin 1g IV q12 hours until delivery
Infant delivers before 36 WGA or before GBS prophylaxis is administered:
Obtain newborn CBC, blood cx
Observe newborn for 48h prior to discharge
Reference: UNC GBS Algorithm
26 y/o G1P0 with protracted labor and rupture of membranes > 18 hours develops acute onset intrapartum fever. Reports chills, increased thirst, dyspnea, dysuria. Epidural anesthesia placed recently. Maternal heart rate > 110 bpm, temperature > 38 C, bilateral pulmonary crackles, costovertebral angle tenderness, abdominal tenderness, uterine tenderness, and malodorous amniotic fluid on exam. IUPC and fetal scalp electrode in place with fetal heart rate > 160 bpm.
Initial Labs
Obtain urinalysis to rule out urinary tract infection
Influenza season and not appropriately vaccinated: Obtain rapid antigen nasopharyngeal influenza swab
Temperature > 39 C
Obtain confirmatory urine culture regardless of urinalysis results
Obtain CBC with differential and evaluate for bandemia indicating acute infection
Consider blood cultures
Concern for intrauterine infection or inflammation
Send amniotic fluid for gram stain, fluid glucose, WBC count, and culture
Send placenta for histopathology
Clinical concern for pneumonia with crackles on exam: Obtain CXR
Treatment
Administer 500 cc LR bolus
Unable to rule out intrapartum infection: Start ampicillin 2g q6h and gentamicin 1.5 mg/kg q8h
Influenza swab positive: Start oseltamivir 75 mg BID x 5 days
CXR positive for PNA
Start azithromycin 500 mg x 1 day followed by 250 mg x 4 days
Not already on ampicillin/gentamicin: Start ceftriaxone 1 g x 5 days
Patient counseled that antibiotic therapy reduces risk of neonatal infection
Notes
Risk factors: Nulliparity, prolonged labor, rupture of membranes > 18 hours
Etiologies
Most common
Epidural anesthesia: Should be suspected only if temperature rose immediately following epidural placement, epidural has been in place less than 4 hours, and the patient has no other signs/symptoms of systemic illness
Intra-amniotic infection: Consider in setting of uterine tenderness and maternal/fetal tachycardia
Respiratory infection
Urinary tract infection
WBC count range for pregnant patients is generally 10,000 to 16,000 and will vary by institution
Fetal heart rate: Category I tracings do not exclude intrauterine infection
Pregnant women
Chorioamnionitis
Labor dystocia
40 y/o G5P4004 at 40 WGA with h/o HTN presents with acute on chronic dyspnea, fatigue. Current pregnancy complicated by pre-eclampsia. Tachycardia, edema on exam.
Obtain CBC, CMP, urine protein/creatinine, LDH, uric acid
EKG shows sinus tachycardia
Echo shows LV dilation/systolic dysfunction and pulmonary hypertension
Treatment
Avoid ACE/ARB, atenolol
Consider HCTZ 25mg qd, metoprolol 12.5mg
Hydralazine 10mg for hypertensive emergency in pregnancy; see preeclampsia with severe features
Titrate diuretics to avoid hypotension, reduced uterine perfusion.
Pt advised that most women recover LV function after pregnancy but that future pregnancies may not be advisable
May develop during 2nd trimester and up to 4 months postpartum
Prevalence ~1:2,500 live births
20 y/o F with h/o obesity, NAFL, HLD presents with irregular menses lasting longer than 6 months. Started menarche more than 2 years ago, denies currently being pregnant, and is currently attempting to conceive. Obesity, terminal hair, alopecia, acne, acanthosis nigricans, and skin tags noted on exam.
Risk factor screening
PHQ-9 positive for depression
STOP-BANG score suggesting sleep apnea
Diagnostic testing
Beta-HCG negative; TSH (N = 0.5-5 mIU/L) and prolactin (N = 2-29 ng/mL) WNL
Total serum testosterone at upper limit of normal (N = 15-70 ng/dL)
Obtain HbA1c, lipid panel
Pelvic U/S shows polycystic ovaries with >12 follicles measuring 2-9 mm
Treatment
Discuss referral to endocrine and starting clomiphene to increase chance of conception success
Start hormonal birth control once pt is no longer attempting to become pregnant
Recommend hair electrolysis vs. laser-based therapy for hair removal
Recommend treating acne with a combination of topical benzoyl peroxide, topical retinoids, and/or topical antibiotics; may consider spironolactone when no longer attempting to conceive
Counseling
Pt counseled about importance of weight loss; calories restricted diet recommended
Pt counseled that her risk for DM type 2 is 4x greater than the general population
Epidemiology/Etiology
Affects approximately 7% of U.S. age females
Insulin resistance may play a role in the pathophysiology of the condition
Diagnosis
Do not start workup within 2 years of menarche as periods are often irregular
Rotterdam criteria for diagnosis: Must meet 2 of 3 findings
Ovulatory dysfunction
Hyperandrogenism (physical exam + serum testosterone)
Polycystic ovaries on U/S
LH:FSH ratio >2 is NOT diagnostic
Consider obtaining TSH, prolactin level, and 17-hydroxyprogesterone level to rule out hypothyroidism, prolactinoma, and/or non-classical congenital adrenal hyperplasia, respectively
If patient meets criteria of ovulatory dysfunction and hyperandrogenism, U/S is not needed to confirm diagnosis
Physical exam
Hirsutism includes terminal hair, alopecia, and acne
Acanthosis nigricans and skin tags are findings indicative of DM
Common comorbidities include obesity, sleep apnea, non-alcoholic fatty liver disease, hyperlipidemia, and depression
Most are benign and found incidentally
Reassure patients, repeat pelvic U/S in 12 months if low risk and
Premenopausal with cyst < 5 cm
Postmenopausal with cyst < 1 cm
Obtain MRI with contrast if unable to reassure (see above) or for patients who do not meet referral criteria (see below); also consider in cysts with features concerning for malignancy
Refer to gynecology for
Symptomatic cysts, e.g. presence of abdominal/pelvic pressure or pain
Cysts > 6 cm
Patients with a family history of breast or ovarian cancer
Presence of intra-abdominal/pelvic ascites
Cysts with
Thick septations (2-3 mm)
Solid regions that are not hyperechoic
Septations or solid regions with blood flow
Elevated CA 125 levels ( i.e. > 35 U/mL)
May correlate with advanced cancer
Should NOT be obtained routinely
Simple ovarian cysts do not increase future risk of malignancy
Median age for diagnosis of ovarian cancer is 63 years
Source: Incidental ovarian cysts: When to reassure, when to reassess, when to refer. Cleveland Clinic Journal of Medicine. 2013 August; 80(8):503-514
Definition: Absence of menarche based presence/absence of secondary sexual characteristics and age
Secondary sexual characteristics present: Age 14 years
Absent: Age 16 years
Etiologies
Gonadal dysgenesis, e.g. Turner syndrome (43% of cases)
Anatomical defects
Mullerian agenesis (10% of cases)
Other: Imperforate hymen, transverse vaginal septum, etc.
Hormonal dysregulation: Hypothalamic amenorrhea, hyperprolactinemia, elevated FSH, polycystic ovary syndrome
Initial workup: Confirm negative beta-hCG and obtain pelvic ultrasound
If no anatomic defects on ultrasound, obtain serum prolactin, FSH, LH, testosterone levels
Consider karyotype based if high suspicion for genetic disorder
Secondary: Defined as cessation of menses for 3 months or irregular menses for 6 months
Most common etiologies
Pregnancy
Primary ovarian failure
Hypothalamic amenorrhea
Hyperprolactinemia
Initial work-up
If pregnancy test negative, obtain TSH, LH, FSH
If visual changes (peripheral vision loss) or galactorrhea present, obtain prolactin level
#Adenomyosis: > 40 y/o F with h/o prior uterine surgery presents with dysmenorrhea. Reports heavy menstrual bleeding and chronic pelvic pain. Diffuse uterine enlargement and uterine tenderness on exam.
Obtain CBC to evaluate for anemia
U/S shows thickened myometrium
Pathology confirms adenomyosis
Pt has not completed childbearing; pt counseled that Mirena IUD may provided limited symptoms relief
Refer to OBGYN for discussion of hysterectomy
#Leiomyoma (fibroids): Pt with h/o [PMH] presents with [timing] [CC]. [HPI]. [PE] on exam.
[Labs]
[Imaging]
[Intervention]
Pt advised to [anticipatory guidance]
#Malignancy/hyperplasia: Pt with h/o [PMH] presents with [timing] [CC]. [HPI]. [PE] on exam.
[Labs]
[Imaging]
[Intervention]
Pt advised to [anticipatory guidance]
#Coagulopathy: Pt with h/o [PMH] presents with [timing] [CC]. [HPI]. [PE] on exam.
[Labs]
[Imaging]
[Intervention]
Pt advised to [anticipatory guidance]
#Ovulatory dysfunction: Pt with h/o [PMH] presents with [timing] [CC]. [HPI]. [PE] on exam.
[Labs]
[Imaging]
[Intervention]
Pt advised to [anticipatory guidance]
#Endometrial etiology: Pt with h/o [PMH] presents with [timing] [CC]. [HPI]. [PE] on exam.
[Labs]
[Imaging]
[Intervention]
Pt advised to [anticipatory guidance]
#Iatrogenic: Pt with h/o [PMH] presents with [timing] [CC]. [HPI]. [PE] on exam.
[Labs]
[Imaging]
[Intervention]
Pt advised to [anticipatory guidance]
15 y/o F with h/o early menarche and heavy menstrual periods presents with pain during first 2-3 days of menses. Pain accompanied by N/V, diarrhea. BMI <20 and normal pelvis on exam.
Obtain U/A, test for G/C
Pending normal results, start naproxen for pain
F/u in 2-3 mo.; consider starting OCPs for refractory pain
30 y/o F with no h/o infertility presents with dysmenorrhea. ROS includes chronic pelvic pain, deep dyspareunia, dyschezia. Pain with lateral cervical movement and a fixed immobile uterus on exam.
Start NSAID + combined OCP
Infertility, contraindications to medical therapy, and desire for definitive diagnosis merit evaluation for laparoscopy; refer to OBGYN
Definition: 45+ y/o F with 12+ months amenorrhea and no alternative biophysiologic explanation
Hot flashes, irregular menses, vaginal dryness, dyspareunia, sleep disturbances, depression/mood change
Labs
TSH if concern for hyperthyroidism
Age < 45 with amenorrhea: Obtain hCG, prolactin, TSH, FSH
Counseling: Expectations per STRAW staging system
Postmenopausal F with h/o chemo/radiation therapy, premature ovarian failure, and oophorectomy presents with insidious onset vaginal itching and clear discharge. Reports dyspareunia. Inflammation and thin, friable mucosa noted on speculum exam.
Start estradiol 10 mcg vaginal insert daily for two weeks and then twice weekly thereafter
Pt advised to use lubricant during sexual activity to reduce discomfort
<10% of vaginitis cases
May also occur in lactating women
Pt with h/o DM and immunocompromised state presents with white vaginal discharge. Reports vulvar itching/burning but denies presence of odor. Medications include corticosteroids and recent course of antibiotics. Vulvar erythema and thick/white/curd-like vaginal discharge on exam.
Hyphae noted on microscopy with KOH prep
Discharge sent for culture
Treatment
Non-pregnant patient
Send two doses of fluconazole 150 mg PO to pharmacy
Administer 1 dose fluconazole today
Pt advised to take second dose in 1 week if symptoms have not resolved
Pregnant patient: Administer miconazole 2% cream 5 g intravaginally daily for 7 days
25% of vaginitis cases
Consider offering prophylactic oral fluconazole when starting women on antibiotics
Complicated vulvovaginal candidiasis
Defined as
Four or more infections in 1 year
Infection in women with poorly controlled DM or AIDS
Severe infection
Send for culture as infection is more likely to be caused by non-albicans Candida
Female pt with h/o smoking, vaginal douching, presents with thin, malodorous discharge that is worse after intercourse. Reports unprotected sexual encounters with multiple sexual partners, including women. Speculum exam reveals thin, homogeneous discharge with fishy odor. Vaginal pH >4.5, positive whiff test and multiple clue cells present on microscopy.

Clue cells indicated by yellow boxes
Start oral metronidazole 500 mg PO BID x 7 days
Pt advised to return for treatment if symptoms recur
Epidemiology
50% of vaginitis cases
Often caused by Gardnerella vaginalis
Higher risk among women who have sex with women
Infected patients are at increased risk for HIV, gonorrhea/chlamydia
Diagnosis based on Amsel criteria
Criteria include
Thin, homogeneous discharge
Vaginal pH >4.5
Positive whiff test with 10% KOH solution
Clue cells on microscopy
3 of 4 criteria required for diagnosis
Pregnancy
Treatment during pregnancy improves symptoms, but does not prevent preterm birth
Vaginal metronidazole can be used in non-pregnant women, but oral metronidazole must be used in pregnancy
Pt with h/o unprotected intercourse with multiple sexual partners, smoking, and recreational drug use presents with acute onset yellow-green, frothy vaginal discharge. Reports vaginal pain/soreness since onset of malodorous discharge. Discharge consistent with pt’s description and strawberry cervix noted on exam.

Trichomonas-related discharge on speculum exam
Microscopy shows motile, flagellated protozoa
Pt is symptomatic and high risk: Obtain trichomoniasis NAAT
Obtain gonorrhea/chlamydia NAAT, rapid plasma reagin (RPR), and 4th generation combination HIV-1/2 immunoassay
Pregnant and non-pregnant patients: Administer metronidazole 2 g PO x 1 dose
Prescribe 1 dose of metronidazole 2 g PO for each of the pt’s recent sexual partners
Pt counseled that active trichomoniasis infection places her at higher risk for preterm labor and contracting HIV
Pt advised to return in 3 months for a test of cure
15% of vaginitis cases
NAAT = nucleic acid amplification test
Presence of trichomoniasis should prompt testing for gonorrhea/chlamydia, syphilis (RPR), and HIV
20 y/o F with h/o repeat gonorrhea/chlamydia infections presents with lower abdominal pain. Reports unprotected sex with multiple partners. Fever, mucopurulent cervical discharge, cervical motion tenderness on exam.
Diagnosis
Perform saline wet mount to evaluate for bacterial vaginosis and trichomonas
Obtain vaginal swab for chlamydia/gonorrhea NAAT, trichomonas NAAT
Obtain syphilis RPR, HBsAG, HIV ELISA
Treatment
Outpatient (empiric):
Ceftriaxone IM 250 mg x 1 dose, doxycycline PO 100 mg BID x 14 days
Add metronidazole PO 500 mg BID x 14 days for any of the following: History of uterine instrumentation within previous 3 weeks, evidence of bacterial vaginosis/trichomonas on exam
Inpatient
Admit to hospital for any of the following reasons: Pregnant, severe abdominal pain, unable to tolerate PO due to vomiting, failure of outpatient therapy, hemodynamic instability (e.g. meets SIRS criteria)
Start cefoxitin IV 2g q6h, doxycycline IV 100 mg q12h and transition to oral therapy after > 24 hours of clinical improvement
Diagnosis
Overall, diagnosis is clinical (81% sensitive)
Ultrasound sensitivity: 30%
Abdominal/pelvic CT sensitivity: Poor
Send vaginal trichomoniasis swab as wet mount sensitivity is poor (51%–65%) versus NAAT (~100%)
Syphilis RPR (as compared to VDRL) reduces false positive results
Additional reading: Acute Pelvic Inflammatory Disease: Diagnostic Performance of CT
18 y/o F presents with new-onset, tender breast mass. Mass size and associated tenderness increase prior to menses. Single, rubbery, mobile, well-circumscribed mobile mass in upper/outer breast quadrant on exam.
Observe for 1-2 menstrual cycles
F/u in 1-2 months; refer for u/s if mass size increases or does not fluctuate with cycles
Pt counseled that mass will likely regress with time
35 y/o F presents with no h/o thyroid disease presents with bilateral, non-bloody nipple discharge. Denies excessive nipple stimulation. Clear discharge is expressed with manipulation; no other abnormalities noted on exam.
Obtain beta hCG, serum prolactin, TSH
Refer for breast u/s and mammogram
Female 6 weeks postpartum presents with focal, unilateral breast tenderness. Reports fever, malaise, nipple soreness, and chronic breast engorgement. Denies shooting pains typically associated with yeast infection. Febrile with peri-areolar skin cracking, erythema, warmth, induration, and pain with palpation on exam.
Evaluate infant for prominent frenulum, cleft palate, thrush
Treatment
Encourage cold compresses and naproxen 500 mg BID for pain
Apply topical mupirocin (Bactroban) 2% ointment to affected area
Start amoxicillin-clavulanate (Augmentin) 875 mg BID x 10 days
Patient failed amoxicillin-clavulanate (Augmentin):
Consider breast milk culture to guide therapy
Sepsis and/or MRSA mastitis: Admit to hospital and start vancomycin 5 mg/kg/dose q12h
Refer for lactation counseling
Counseling
Pt encouraged to continue feeding with both breasts during treatment
Pt advised to perform frequent, complete emptying of the breast to prevent abscess formation
Bilateral erythema decreases likelihood of infectious etiology
Poor breast drainage increases risk for infection and abscess formation
Obtain breast milk culture for
Failed response to initial treatment
Hospital acquired mastitis
Severe infections (e.g sepsis)
Yeast infection treatment
Mother: Fluconazole 400 mg on day 1 followed by 200 mg daily for 10+ days
Infant: Fluconazole 20 mg/kg on day 1 followed by 5 mg/kg for 10+ days